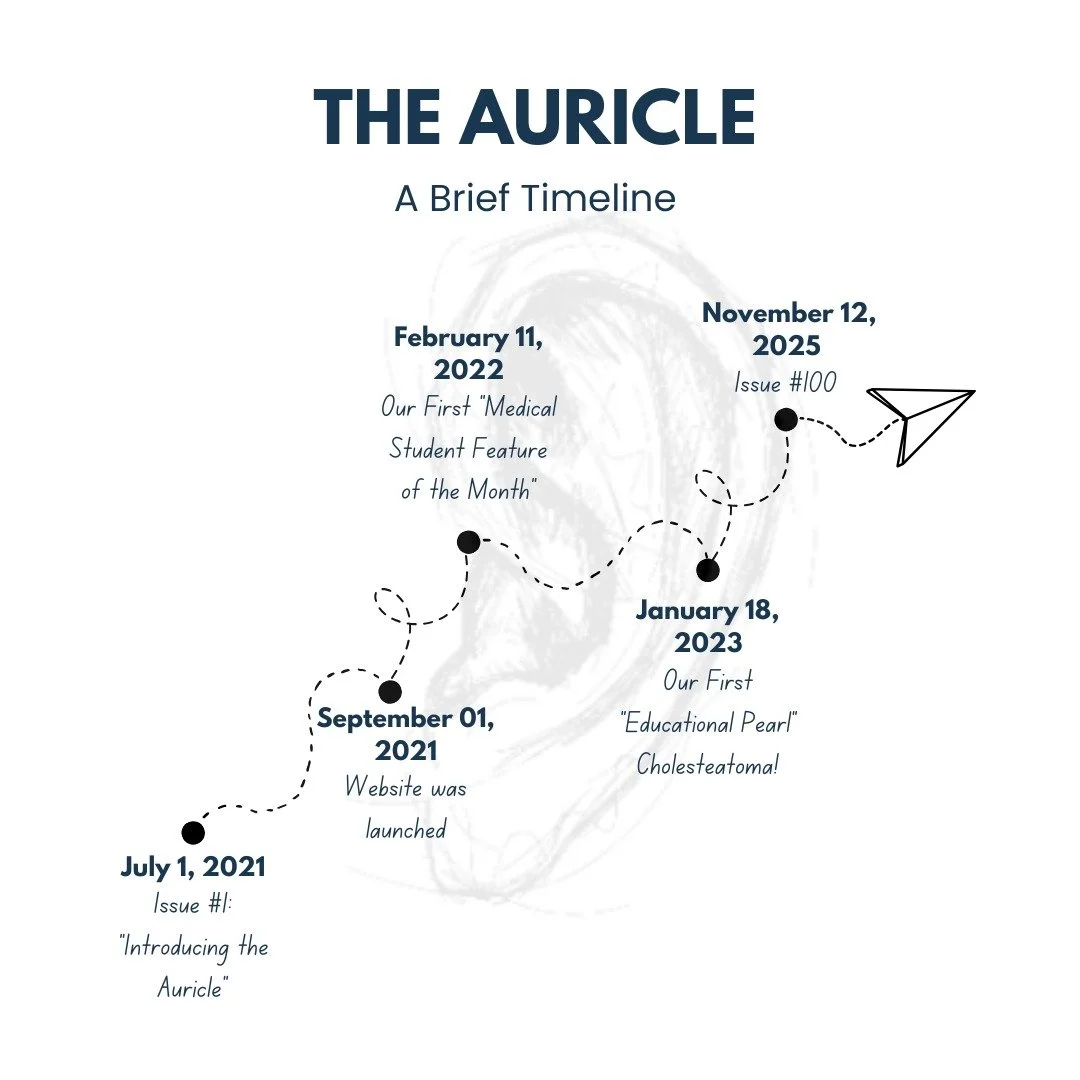
Issue #104
23 January 2026
A medical student-run newsletter showcasing the latest cutting edge research in otolaryngology
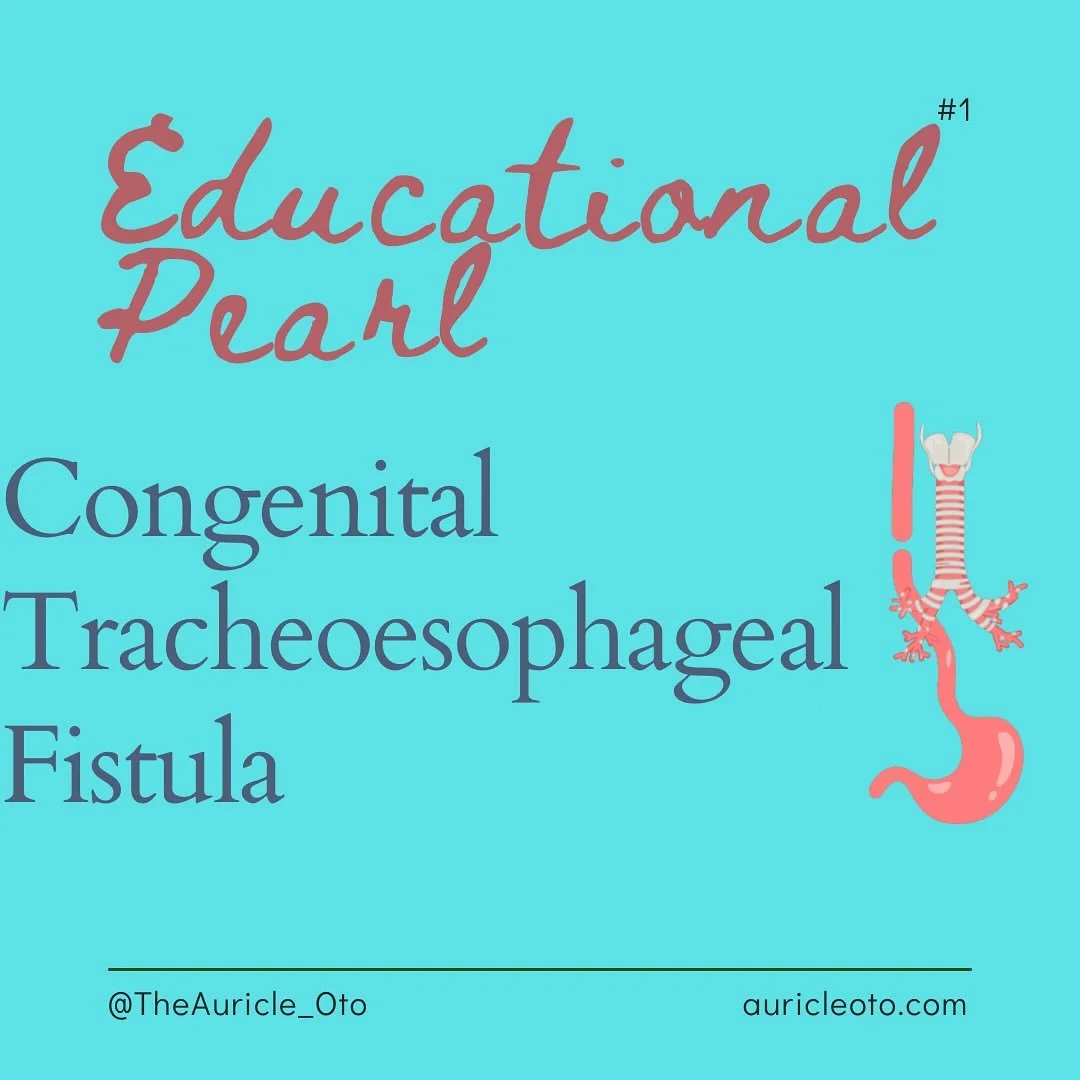
Educational Pearl
Obstructive Sleep Apnea
Overview: Obstructive sleep apnea (OSA) is a sleep-related breathing disorder caused by recurrent partial or complete upper airway collapse during sleep, leading to intermittent airflow limitation, hypoxemia, and sleep fragmentation. Untreated OSA is associated with significant cardiovascular and cerebrovascular morbidity, often driven by nasal, palatal, tonsillar, or craniofacial obstruction.
Epidemiology:
Adults:
U.S. Prevalence: 33.9% in men, 17.4% in women
Risk Factors: Older age, male sex, lower socioeconomic status, smoking, prematurity
Children:
Prevalence: 2-5%, higher in select clinical populations
Peak Incidence: Ages 2-8 years old (adenotonsillar hypertrophy)
Increased risk with craniofacial abnormalities (Crouzon, Pierre Robin, Apert, cleft lip or palate)
Comorbidities: Hypertension, mood disorders, cognitive dysfunction, acute coronary syndromes, congestive heart failure, atrial fibrillation, type 2 diabetes
Etiology:
Partial or complete upper airway collapse during sleep
Recurrent arousals and/or oxygen desaturation ≥ 3%
Contributing factors can be anatomic, physiologic, genetic, environmental, and/or neuromuscular
Clinical Presentation:
Adults: Excessive daytime sleepiness, nonrestorative sleep, insomnia, nocturnal choking/gasping/breath holding, loud snoring, witnessed apneas, morning headaches
Children: Behavioral changes (hyperactivity, irritability, aggression), snoring, mouth breathing, witnessed apneas, nighttime awakenings, secondary nocturnal enuresis
Diagnosis:
Clinical suspicion based on history and symptoms
Physical Examination:
BMI, neck circumference
Macroglossia, tonsillar size (Mallampati score)
Enlarged uvula, retrognathia
Nasal septal deviation, turbinate hypertrophy
Screening Questionnaires:
Epworth Sleepiness Scale
Berlin Questionnaire
STOP-BANG
Nasopharyngeal endoscopy if adenoidal hypertrophy is suspected
Polysomnography:
≥ 5 obstructive events per hour with symptoms and/or comorbidities, or
≥ 15 obstructive events per hour regardless of symptoms
Severity defined by apnea-hypopnea index (AHI)
Management:
Medical and Adjunctive: Optimize modifiable risk factors
Adults: Continuous positive airway pressure (CPAP), positional therapy, weight loss, oral appliances or mandibular repositioning devices, avoid alcohol/sedatives before sleep
Children: Intranasal corticosteroids for mild-to-moderate disease
Surgical: Address underlying structural abnormalities
Adults: Uvulopalatopharyngoplasty, maxillomandibular advancement, upper airway stimulation, septoplasty, tracheostomy (select cases)
Children: Adenotonsillectomy, inferior turbinate reduction
Further Readings:
[1] OSA Diagnosis and Management
[2] StatPearls - Pediatric OSA
Educational Pearl written by Gina Spencer
Queen's University School of Medicine
Question of the Week
A 54-year-old woman presents with progressive unilateral hearing loss, intermittent tinnitus, and gait instability that worsens in the dark. Audiometry shows asymmetric sensorineural hearing loss, greatest at high frequencies. Brain MRI with contrast reveals no cerebellopontine angle mass but demonstrates endolymphatic hydrops with subtle enhancement near the fundus of the internal auditory canal.
Which of the following is the most appropriate next diagnostic step to determine the underlying pathology?
(A) Auditory brainstem response testing to evaluate retrocochlear conduction delays
(B) Vestibular evoked myogenic potentials to assess superior vs. inferior vestibular nerve involvement
(C) Electrocochleography to assess summating potential-to-action potential ratios
(D) High-resolution MRI with internal auditory canal protocol focusing on the fundus
(E) CT of the temporal bone to evaluate otic capsule demineralization
Question of the Week written by Luke Reardon
Lincoln Memorial University DeBusk College of Osteopathic Medicine
· · · · · · · · · · · · · · · · · · · · · · · · · · · · ·
Want the Question of the Week answer?
Find it at the bottom of this newsletter!
The high-quality otolaryngology content we deliver each month is made possible of our national faculty reviewers. We sincerely thank them for their contributions to The Auricle.
Facial Plastic and Reconstructive Surgery
Dr. Leslie Kim, MD, MPH
The Ohio State University Wexner Medical Center
Head and Neck Surgery
Dr. Michael Topf, MD
Vanderbilt University Medical Center
Laryngology
Dr. Karuna Dewan, MD
Louisiana State University Health
Otology and Neurotology / Basic Science Spotlight
Dr. Emily Stucken, MD
University of Michigan Medical Center
Pediatric Otolaryngology / Med Student Feature
Dr. Michele Carr, MD, DDS, PhD
Jacobs School of Medicine, University at Buffalo
Rhinology and Skull Base Surgery
Dr. Christina Fang, MD
Montefiore Medical Center
Med Student Feature Series
This series showcases student-led research published in leading otolaryngology journals, highlighting evidence-based discoveries from the next generation of otolaryngologists.
Pooja Reddy
University of Pittsburgh School of Medicine
Class of 2026
Turbinate Reduction and Pediatric OSA Outcomes
Reddy PD, Ung S, Qian J, et al. Does Inferior Turbinate Reduction Improve Outcomes Following Adenotonsillectomy in Pediatric OSA and Allergic Rhinitis Patients? Int J Pediatr Otorhinolaryngol. 2025;198:112578. [Article Link]
Breathe better, sleep better
Children with sleep disordered breathing (SDB) and allergic rhinitis (AR) often continue to experience nasal obstruction after adenotonsillectomy (TA), yet few studies have examined whether adding inferior turbinate reduction (ITR) improves outcomes in patients who have both conditions. To address this gap, this retrospective cohort study assessed 269 pediatric patients with confirmed SDB and AR for symptom and medication use changes after TA alone (N = 227, 84.4%) or TA with ITR (N = 42, 15.6%). Group sample sizes varied in specific analyses because not all symptoms or medications were documented at both pre- and post-operative visits, resulting in smaller denominators for each comparison. Both groups experienced significant reductions in nasal congestion postoperatively, decreasing in frequency from 77.6% (N = 156) to 23.9% (N = 48) of patients in the TA alone group and from 78.9% (N = 30) to 39.5% (N = 15) of patients in the TA with ITR group (χ² = 91.1 and χ² = 10.7, respectively; both p < .001). The presence of snoring also declined significantly in both groups after surgery, from 97.0% (N = 197) to 11.8% (N = 24) of TA alone patients and from 97.4% (N = 37) to 10.5% (N = 4) of TA with ITR patients (χ² = 81.0 and χ² = 33.0; both p < .001). Nasal corticosteroid use decreased in both groups as well, from 32.8% (N = 66) to 21.4% (N = 43) of TA alone patients and 80.5% (N = 33) to 41.5% (N = 17) of TA with ITR patients (χ² = 9.28 and χ² = 14.2, respectively; both p ≤ .002). Because the study did not compare postoperative outcomes between groups, the findings support both surgeries as effective options and suggest that surgeons should individualize ITR addition decisions based on patient-specific anatomy and symptom severity rather than presumed added benefit.
Medical Student Feature Summary written by Anders Erickson
Des Moines University College of Osteopathic Medicine
Are you a medical student with a recent first-author publication?
Email theauricleotolaryngology@gmail.com to be featured!
Facial Plastic and Reconstructive Surgery
AI for Patient-Initiated Contact After Rhinoplasty
Palmer WJ, Michlin D, Estephan L, et al. Utility and Safety of Artificial Intelligence for Patient-Initiated Contact After Functional Rhinoplasty. OTO Open. 2025 Dec;9(4):70170. [Article Link]
Can a chatbot answer your post-op rhinoplasty call?
Postoperative patient-initiated contact is common after functional rhinoplasty and contributes substantially to provider workload, yet most concerns are low acuity and reassurance-based. This study evaluated whether widely available artificial intelligence (AI) chatbots can safely address common postoperative questions, including potential “red flag” symptoms. In a single-institution retrospective review of 378 patients undergoing functional rhinoplasty (2017 to 2023) postoperative calls/messages prior to first follow-up were distilled into 48 representative prompts (including six red-flag questions) and entered into four AI chatbots (ChatGPT, Claude, Perplexity, Gemini), with responses graded by two blinded experts using a Global Quality Scale, binary Expert Opinion Question, and Flesch-Kincaid (FK) readability scores. Of 378 patients, 137 (36.2%) initiated postoperative contact a total of 181 times, most commonly for pain (N = 181, 19%), medication questions (N = 181, 14%), and congestion (N = 181, 13%); 72.9% (132/181) of patients were managed with routine counseling and had no complications at first follow-up. ChatGPT produced “good” or “excellent” responses 98% of the time, significantly outperforming Perplexity (79%; p = .0039), and was unanimously approved by experts in 96% of cases and 100% of red-flag scenarios. Readability remained suboptimal across all chatbots, with ChatGPT scoring at the highest grade level (median FK grade level 16.8) and Perplexity the lowest (13.2; p < .0001). AI chatbots may serve as effective first-line tools for managing low-acuity postoperative concerns after functional rhinoplasty, potentially reducing staff workload without compromising patient safety, though improvements in readability and careful clinician vetting of AI platforms are essential before broader clinical integration.
Facial Plastic and Reconstructive Surgery Summary written by Nicole Reynoso
University of California, San Francisco School of Medicine
Head and Neck Surgery
Serum Metabolics for Oral Cavity Cancer Prognosis
Shen EY, Lee LY, Ng SH, et al. Serum Metabolomics for Prognostic Stratification in Resected Advanced-Stage Oral Cavity Cancer. JAMA Otolaryngol Head Neck Surg. Published online December 4, 2025. [Article Link]
Current staging and risk assessment tools remain limited in predicting recurrence in advanced-stage oral cavity squamous cell carcinoma (OCSCC), highlighting the need for improved prognostic methods. This single-institution retrospective cohort study evaluated whether preoperative serum metabolomics could predict recurrence and survival in 228 treatment-naïve patients with resected advanced-stage OCSCC (216/228, 94.7% male; mean age 51.9 ± 10.8 years) treated between 2007 and 2018 (mean follow-up 86 ± 51 months), using liquid chromatography–mass spectrometry to identify 19 serum metabolites associated with adverse disease-free survival (DFS) and develop the MetaboScore, which stratified patients into low-risk (< 11; N = 149, 65.4%) and high-risk (≥ 11; N = 79, 34.6%) groups. Compared with low MetaboScores, high MetaboScores were associated with higher rates of local recurrence (22/79, 27.8% vs. 5/149, 3.4%), regional recurrence (19/79, 24.1% vs. 10/149, 6.7%), and distant metastasis (29/79, 36.7% vs. 21/149, 14.1%). Five-year DFS was significantly worse in the high-risk group (38%, 95% confidence interval [CI] 28 to 51 vs. 83%, 95% CI 77 to 89; absolute difference 45 percentage points, 95% CI 32 to 57), as was disease-specific survival (58%, 95% CI 48 to 70 vs. 85%, 95% CI 79 to 91; absolute difference 27 percentage points, 95% CI 14 to 40). On multivariable analysis, a MetaboScore ≥ 11 independently predicted worse local control (hazard ratio [HR] = 14.8, 95% CI 5.5 to 40.4), neck control (HR = 4.2, 95% CI 1.9 to 9.2), DFS (HR = 4.4, 95% CI 2.7 to 7.2), and disease-specific survival (HR = 3.0, 95% CI 1.8 to 5.3). Among patients with pN3b disease (N = 72, 31.6%), a MetaboScore ≥ 11 further stratified outcomes, with worse five-year DFS (20% vs. 60%) and disease-specific survival (28% vs. 62%), demonstrating prognostic value beyond American Joint Committee on Cancer staging alone. These findings suggest that serum metabolomic profiling may enhance postoperative risk stratification in OCSCC, though prospective validation in more diverse populations is required before clinical implementation.
Head and Neck Surgery Summary written by Brooke Swain
Vanderbilt University School of Medicine
Laryngology
GLP-1 Receptor Agonists for Chronic Cough
Gallagher TJ, Razura DE, Li A, Kim I, Vukkadala N, Barbu AM. Glucagon-Like Peptide-1 Receptor Agonists and Chronic Cough. JAMA Otolaryngol Head Neck Surg. Published online November 26, 2025. [Article Link]
Slowed gastric emptying, new cough: The GLP-1RA connection
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are increasingly prescribed for type 2 diabetes (T2D) and obesity and delay gastric emptying, raising concern for reflux-related effects. Chronic cough is common and multifactorial, and although reflux is a well-recognized contributor, many patients have refractory or unexplained symptoms, suggesting possible medication-related causes. This multicenter retrospective cohort study used the TriNetX research network to compare adults with T2D prescribed GLP-1RAs with those prescribed other second-line diabetes medications in a cohort of 427,555 patients. After propensity score matching, outcomes included new-onset chronic cough and new gastroesophageal reflux disease (GERD) diagnoses within five years of medication initiation. GLP-1RA use was associated with a higher risk of new chronic cough compared with non-GLP-1RA therapy (adjusted hazard ratio [aHR] = 1.1, 95% confidence interval [CI] 1.2 to 1.2), including dipeptidyl peptidase-4 inhibitors (aHR = 1.2, 95% CI 1.1 to 1.3) and sulfonylureas (aHR = 1.3, 95% CI 1.2 to 1.3), with no significant difference compared with sodium-glucose cotransporter 2 inhibitors. Among patients without any diagnosis of GERD, the association between GLP-1RA use and chronic cough was stronger (aHR = 1.3, 95% CI 1.2 to 1.4), suggesting mechanisms beyond GERD alone. These findings support an association between GLP-1RA use and chronic cough that may reflect reflux-related or neurogenic pathways and highlight the importance of considering GLP-1RA exposure when evaluating patients with refractory or unexplained cough.
Laryngology Summary written by Emily Chestnut
Indiana University School of Medicine
Otology and Neurotology
Results of New Semi-Synthetic TORP Ossiculoplasty
Malafronte G, Trusio A, Rossetti V, Colacurcio V, De Cristofaro G, Filosa B. New Semi-Synthetic TORP Ossiculoplasty: Long-Term Results. Otol Neurotol. Published online November 19, 2025. [Article Link]
Total ossicular replacement prosthesis (TORP) ossiculoplasty is a surgical procedure that aims to reconstruct the ossicular chain in the mammalian ear with the goal of restoring conductive hearing loss. Although TORP ossiculoplasty has evolved significantly since the 1990s, its use in clinical practice has declined in recent years. Given the variability in the TORP ossiculoplasty procedure, the authors share long-term results of their novel semi-synthetic TORP named the Malafronte TORP (MTORP) that uses perichondrium-covered cartilage at the head and base to improve long-term coupling and stability, key areas in which prior prosthesis were lacking. In this prospective study, 18 total columellar ossiculoplasties using MTORP positioned between the tympanic membrane and the stapes footplate (with or without a stapes superstructure) were conducted with short-term (mean 8.5 months) and long-term (mean 24.5 months) follow-up. With the primary outcome being postoperative air-bone gap (ABG), short-term follow-up revealed a decrease in mean ABG from 33.5 ± 8.7 dB preoperatively to 12.2 ± 6.0 dB, with 88.8% (16/18) achieving an ABG ≤ 20 dB (t = 27.7; p < .05). At long-term follow-up, the mean ABG was 13.2 ± 5.5 dB with 83.3% (15/18) achieving an ABG ≤ 20 dB (t = 15.3; p < .05 vs. preoperative), with no statistically significant difference present between short and long-term ABG (t = 0.45; p > .05) and a 0% extrusion rate. These results suggest that the novel MTORP cartilage-perichondrium interface may serve as a useful mechanism for durable integration and reduced extrusion risk, although more comparative studies and longer term surveillance are required before this technology can be broadly adopted.
Otology and Neurotology Summary written by Maaz Haji
Chicago Medical School, Rosalind Franklin University
Pediatric Otolaryngology
Pediatric Mastoiditis and Intracranial Complications
Hess-Erga J, Dyrhovden GS, Engesæter I, et al. C-reactive Protein as a Predictor of Intracranial Complications in Paediatric Acute Mastoiditis: Findings From a 25-Year Retrospective Study. Int J Pediatr Otorhinolaryngol. 2026 Jan;200:112674. [Article Link]
Acute mastoiditis (AM) is a potentially serious complication of acute otitis media in children with a risk of intracranial complications despite modern antibiotic therapy. This 25-year retrospective study examined treatment pathways and outcomes of pediatric AM cases to identify clinical and laboratory predictors of severe disease, such as C-reactive protein (CRP) and white blood cell (WBC) count, to improve risk stratification. Researchers performed a single-center retrospective review at a Norwegian tertiary hospital between 2000 and 2024, including all pediatric patients (< 18 years) with a diagnosis and treatment of AM and excluding those with external ear infections, cholesteatomas and infections due to grommets, analyzing clinical presentation, computed tomography findings, laboratory values, treatment, and outcomes. Statistical comparisons were made between uncomplicated cases and those with intracranial complications (epidural or cerebellar abscess and/or sigmoid sinus thrombosis [SST]). Among 143 children (N = 86, 60.1% male; N = 57, 39.9% female), 7.0% (N = 10) developed intracranial complications (epidural abscess: 6/143, 4.2%; SST: 3/143, 2.1%), and these patients had significantly higher CRP levels (mean 236 ± 76.8 mg/L, range 157 to 358 mg/L) at admission compared to those without intracranial disease (mean 110 ± 89.6 mg/L, range 1 to 426 mg/L; p < .001). WBC count was not significantly different between patients with and without intracranial abscesses (p = .400). This study highlights that markedly elevated CRP in a child with suspected acute mastoiditis should raise concern for intracranial complications and prompt a lower threshold for early imaging and aggressive management.
Pediatric Otolaryngology Summary written by Gabriella Adams
Eastern Virginia Medical School
Rhinology and Skull Base Surgery
GLP-1R Agonists in Post-Sinus Surgery Management
Hoying D, Kaelber DC, Chaaban MR. Assessing the Impact of GLP-1R Agonists in Post-Sinus Surgery Management. Otolaryngol Head Neck Surg. 2025;00(00):1–6. [Article Link]
Can GLP-1 medications reduce the need for revision sinus surgery?
Obesity has been increasingly implicated in chronic rhinosinusitis with nasal polyps (CRSwNP), yet the effect of weight-loss therapies on postoperative sinus surgery outcomes remains unclear. This study aimed to evaluate whether glucagon-like peptide-1 receptor agonist (GLP-1RA) use is associated with reduced revision surgery and postoperative biologic therapy following functional endoscopic sinus surgery (FESS) in obese patients with CRSwNP. In this retrospective cohort study using the TriNetX Analytics platform, adult patients with obesity, CRSwNP, and at least one prior FESS were analyzed, comparing those prescribed GLP-1RAs with those who were not. After 1:1 propensity score matching, 1,391 GLP-1RA patients were compared with 1,391 matched non-GLP-1RA controls, with outcomes including revision FESS and first-time biologic prescription at one- and five-year follow-up. GLP-1RA use was associated with significantly lower revision FESS rates at one year (33/1391, 2.4% vs. 52/1391, 3.7%; relative risk [RR] = 0.64, 95% confidence interval [CI] 0.41 to 0.98; p = .04) and five years (61/1391, 4.4% vs. 102/1391, 7.3%; RR = 0.60, 95% CI 0.44 to 0.81; p = .001). While no significant difference in biologic initiation was observed at one year, GLP-1RA patients had reduced first-time biologic prescriptions at five years (60/1206, 5.0% vs. 92/1326, 6.9%; RR = 0.72, 95% CI 0.52 to 0.98; p = .04). These findings suggest that GLP-1 receptor agonists may impact long-term disease recurrence after sinus surgery, but prospective studies are needed to clarify their role in postoperative management.
Rhinology and Skull Base Surgery Summary written by Rushi Vekariya
University of Central Florida College of Medicine
Basic Science Spotlight
Trackerless Navigation in Trans-Mastoid Surgery
Bartholomew RA, Zhou H, Mital K, et al. Integration of Trackerless Surface Reconstruction-Based Surgical Navigation With Exoscopic Trans-Mastoid Surgery. Otolaryngol Head Neck Surg. Published online November 12, 2025. [Article Link]
Lateral skull base surgery requires millimeter-level precision, yet existing navigation systems are underused due to workflow disruption and reliance on external tracking hardware. This interventional cadaveric study evaluated the feasibility and accuracy of a trackerless simultaneous localization and mapping (SLAM) based navigation system during exoscopic trans-mastoid surgery in 10 deceased donor temporal bones by coregistering high fidelity surface models to preoperative CT imaging which allows for representation of the relationship between the exposed tissue surface and underlying anatomy. Following successful surface reconstruction and model alignment, high-fidelity surface reconstruction was achieved, and produced reliable co-registration. Mean reconstruction error was 0.72 ± 0.32 mm, and the mean registration error was 1.43 ± 0.49 mm. Sufficient accuracy was maintained during surgical progression with exoscope-exoscope surface-surface error of 1.72 ± 0.59 mm, and during transitions between visualization modalities. Overall, the authors conclude that exoscope-integrated, trackerless SLAM-based navigation enables accurate and continuous intraoperative guidance without external tracking hardware, supporting its great potential in improving efficiency and ergonomics in lateral skull base surgery with further clinical validation.
Basic Science Spotlight Summary written by Sue Li
Texas Tech University School of Medicine
Question of the Week
Correct Answer and Explanation
Correct Answer: (D) High-resolution MRI with internal auditory canal protocol focusing on the fundus
Answer Explanation: This patient’s progressive unilateral sensorineural hearing loss, intermittent tinnitus, and imbalance strongly suggest a retrocochlear process, most concerning for vestibular schwannoma. Audiometry demonstrating asymmetric high-frequency hearing loss further supports retrocochlear pathology. Although endolymphatic hydrops is noted on imaging and may contribute to vestibular symptoms and tinnitus, it does not explain progressive asymmetric high-frequency hearing loss or focal enhancement at the fundus of the internal auditory canal (IAC). Very small vestibular schwannomas may be confined to the IAC fundus and can be missed on standard brain or cerebellopontine angle MRI sequences. Therefore, the most appropriate next diagnostic step is answer choice (D), high-resolution, thin-cut MRI with a dedicated IAC protocol to fully evaluate the fundus and confirm or exclude an early intracanalicular vestibular schwannoma.
Answer choice (A), auditory brainstem response testing, may detect larger retrocochlear lesions but has reduced sensitivity for small intracanalicular tumors and may be normal early in the disease course. MRI with IAC protocol is the gold standard in this context. Answer choice (B), vestibular evoked myogenic potentials, evaluates vestibular pathway function but cannot reliably distinguish endolymphatic hydrops from a small fundal schwannoma. Answer choice (C), electrocochleography, is useful for diagnosing endolymphatic hydrops but does not clarify the significance of focal IAC enhancement or adequately assess for vestibular schwannoma. Answer choice (E), CT of the temporal bone, is best for bony pathology such as otosclerosis and provides poor soft-tissue resolution for small intracanalicular lesions, making it inappropriate in this setting.
Sources:
[1] Pasha R, Golub JS. Otolaryngology Head and Neck Surgery: Clinical Reference Guide. 4th ed. Plural Publishing; 2014.
[2] AAO-HNSF. Primary Care Otolaryngology Handbook. 4th ed. Alexandria, VA: American Academy of Otolaryngology-Head and Neck Surgery Foundation; 2019:70-76.
Question of the Week Answer written by Luke Reardon
Lincoln Memorial University DeBusk College of Osteopathic Medicine
Special Podcast Feature: Sundays with Saima & Co.
The Auricle on Social Media